
Overview
HCT/Ps are defined as; “articles containing human cells or tissues that are intended for implantation, transplantation, infusion, or transfer into a human recipient” by Food and Drug Administration (FDA) and include:
-
Musculoskeletal tissue and skin
-
Ocular tissue
-
Cellular therapies
-
Hematopoietic stem/progenitor cells
-
Therapeutic cells (DLI)
-
Somatic cells (regardless of source)
-
Reproductive tissue
-
Combination tissue/device, tissue/drug
-
Human heart valve allografts
-
Human Dura mater
Regenerative medicine and (stem) cell therapy is a group of new techniques, or technologies, that rely on replacing diseased or dysfunctional cells with healthy, functioning ones. It also allows scientists to repair damaged cell, tissue and organ. Several disorders are candidate for cellular therapy for instance; Cancers, Diabetes Mellitus, Neurological disorders, Cardiovascular, Autoimmune, Musculoskeletal diseases, Rheumatoid arthritis, etc. Various (stem) cell types have been evaluated for the mentioned applications in basic, experimental, and also clinical trial researches (e.g. mesenchymal stem cell, hematopoietic stem cell, embryonic stem cell, and etc.).
Considering safety concerns regarding regenerative medicine and cellular therapy, HCTPs has been divided into two groups by FDA:
Lower risk- Autologous or family related donors and minimally manipulated and homologous use (GTP compliance is enough for manufacturing this type of products)
Higher risk- Allogeneic unrelated donors and/or more than minimally manipulated and/or non-homologous use (both of GTP and GMP compliance are needed)
 GLP- Good Laboratory Practice
GLP- Good Laboratory Practice
 GTP- Good Tissue Practice
GTP- Good Tissue Practice
 GMP- Good Manufacturing Practice
GMP- Good Manufacturing Practice
 GCP- Good Clinical Practice
GCP- Good Clinical Practice
Objective
This course is established upon the scientific experience and expertise of professionals to provide trainees with valuable skills and experience in this area of science. It is expected to cover suitable issues regarding regenerative medicine and cellular therapy for trainees and their Institutes.
Methodology
The course will provide participants with the knowledge, skills, and hand-on technical expertise necessary to face the challenges of manufacturing HCTPs for basic research or clinical trials. It consists of HCTPs manufacturing standards and guidelines, theoretical knowledge and also practical skills in the field of regenerative medicine. It is to be taught in two modules:
Theoretical modules (on-line training)
o Basic cell culture techniques
o Aseptic techniques and working in controlled environment
o Cell manufacturing for clinical applications
o Pre-clinical studies and clinical trial design
These modules include different sub-topics as below,
a. Basic physiology of cells, tissues and organs
b. Current status of (stem) cell and tissue engineering research
c. Translational sciences in regenerative medicine
d. Animal modeling and preclinical animal studies
e. Regenerative medicine and therapeutic potential of cell and tissue products
f. Cell and tissue manufacturing methodology
g. Basic principles of cell and tissue banking
h. International standards and guidelines
i. Safety concerns
j. Ethical and legal issues
k. Quality management systems
l. Differences between clinical and non-clinical manufacturing of HCTPs
m. GLP, GTP, and GMP complaint HCTPs
n. Risk management related to HCTPs
o. Clinical trial design (GCP) in regenerative medicine
p. Current state and future perspective of regenerative and HCTPs-based therapy
q. Different sources of stem cells for research and clinical application
r. Extra-embryonic tissue derived (stem) cells (Dr. Ornella Parolini).
ü Placental derived stem cells and their application
ü Epithelial and mesenchymal (stem) cells from the amniotic membrane
Practical training
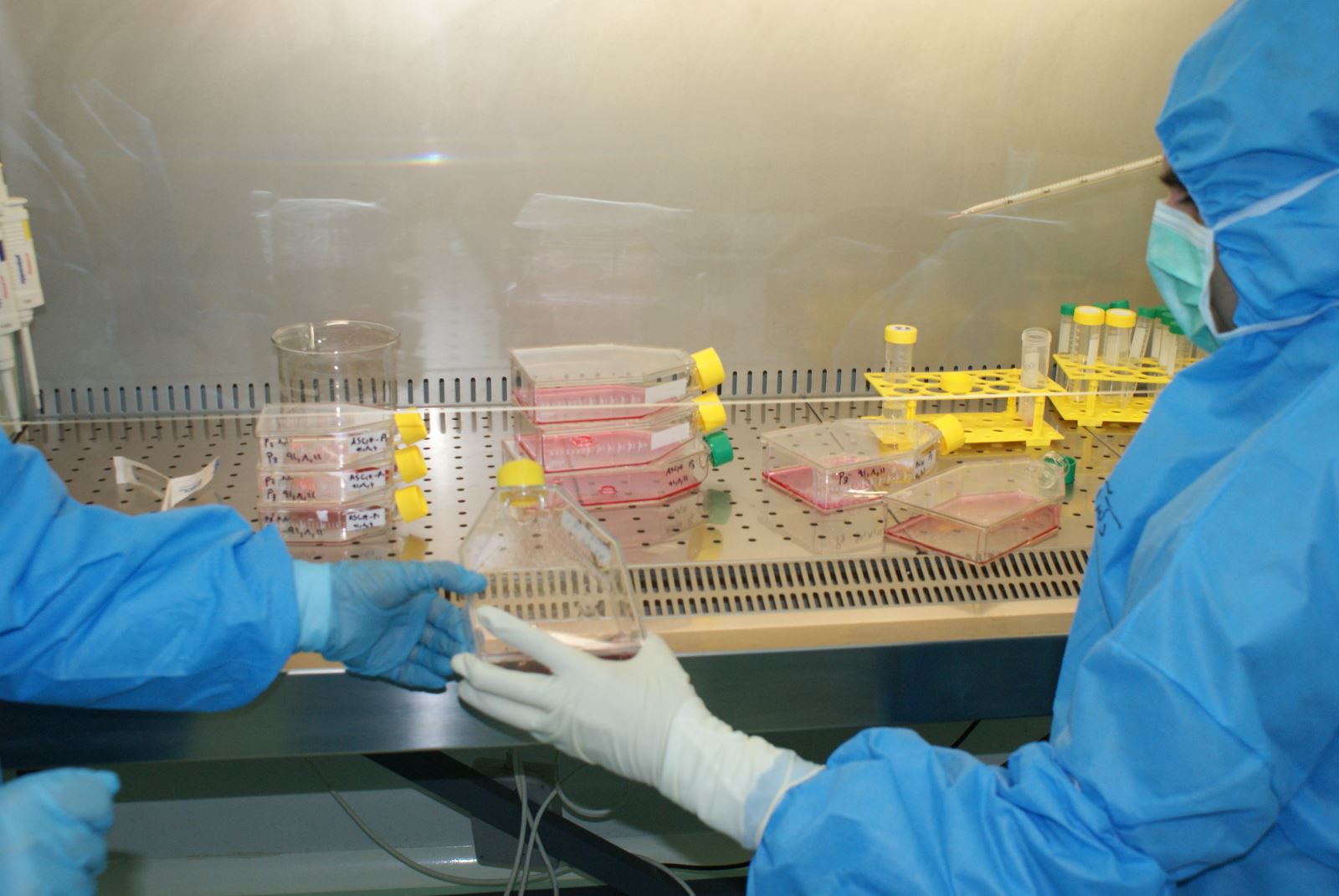
o Essentials of practice in fully equipped GMP facility (clean room)
o Stem cell manufacturing techniques
Bone marrow derived mesenchymal stem cell
Adipose tissue derived stem cells
Extra-embryonic tissue derived (stem) cells as a novel source (Dr. Ornella Parolini)
Placental derived stem cells
Amnion derived epithelial and mesenchymal stem cells
o Magnetic cell sorting
o Stem cell freezing and banking
o Working with equipments and instruments in these facilities
o Environmental control, documentation
Who can attend?
The course is intended to train biomedical students (B.Sc., M.S., and Ph.D. students) and professionals who are working in the field of (stem) cell research and therapy such as:
Cell culture staff
Tissue Bankers
Quality control staff
Other professionals from various relevant background who are seeking such courses
Course duration
The course lasts 1year and contains theoretical modules (web-based) and 3 Weeks workshop (in-site training)
Application requirements
The applicants are requested to submit TUMS online application form to apply for this position.
5’000 $ payment
The language of instruction for this course is English.
TUMS international students will be given the opportunity to use our international dormitories located close to campus in Tehran.
For more information please read TUMS admission criteria and guidelines.
Organizer: Office of Vice-Chancellor for Global Strategies and International Affairs, Tehran University of Medical Sciences (TUMS)
Conducted by: Brain and Spinal Injury Research Center affiliated to TUMS (Clinical grade stem cell manufacturing facilities)
Course directors:
Dr. Babak Arjmand, M.D., M.Phil, Ph.D., Managing Director of Clinical and GMP grade cell and tissue manufacturing facilities, Brain and Spinal Injury Research Center, Tehran University of Medical Sciences, Tehran, Iran
Dr. Hamidreza Aghayan, M.D., M.Phil, Ph.D., Medical Director of Clinical and GMP grade cell and tissue manufacturing facilities, Brain and Spinal Injury Research Center, Tehran University of Medical Sciences, Tehran, Iran
Visiting professor:
Dr. Ornella Parolini, Regenerative Medicine Research Center, Fondazione Poliambulanza-Instituto Ospelaliero, Brescia, Italy
Advisory board:
Dr Stephen Strom, Ph.D., Professor, Cell Transplantation and Regenerative Medicine, Department of Laboratory Medicine, Division of Pathology, Karolinska Institutet, Stockholm, Sweden
Dr Ahmadreza Dehpour, Ph.D., Professor, Department of Pharmacology, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
Dr Masoud Soleimani, Ph.D., Associate Professor, Department of Hematology and Blood Banking, Tarbiat Modares University, Tehran, Iran
Dr Mohammadtaghi Joqhataei, Ph.D., Professor, Cellular and Molecular Research Center, Iran University of Medical Sciences, Tehran, Iran
Dr Gholamreza Pourmand, M.D., Professor, Urology Research Center, Tehran University of Medical Sciences, Tehran, Iran
Dr Ali Jafarian, M.D., Associate Professor, Hepatobiliary Surgery & Liver Transplantation Division, Department of General Surgery, Imam Khomeini Hospital, Tehran University of Medical Sciences, Tehran, Iran
Dr Mohsen Nasiri-toosi, Associate Professor, Department of Gastroenterology, Imam Khomeini Hospital, Tehran University of Medical Sciences, Tehran, Iran